Research
We are developing new chemical reactions by integrating different fields such as chemistry, pharmacology, medicine, and agriculture, using photochemistry as a key. For example, we are investigating chemical reactions to convert dairy-derived biogas into liquid energy such as methanol and formic acid through the photochemistry of chlorine dioxide, and we are researching new therapeutic agents using catalysts that absorb specific light.
Development of chemical reactions using chlorine dioxide
Methanol synthesis from biogas
 Ohkubo, K.; Hirose, K. Angew. Chem. Int. Ed. 2018, 57, 2126
Ohkubo, K.; Hirose, K. Angew. Chem. Int. Ed. 2018, 57, 2126Chemical conversion of biogas into useful substances
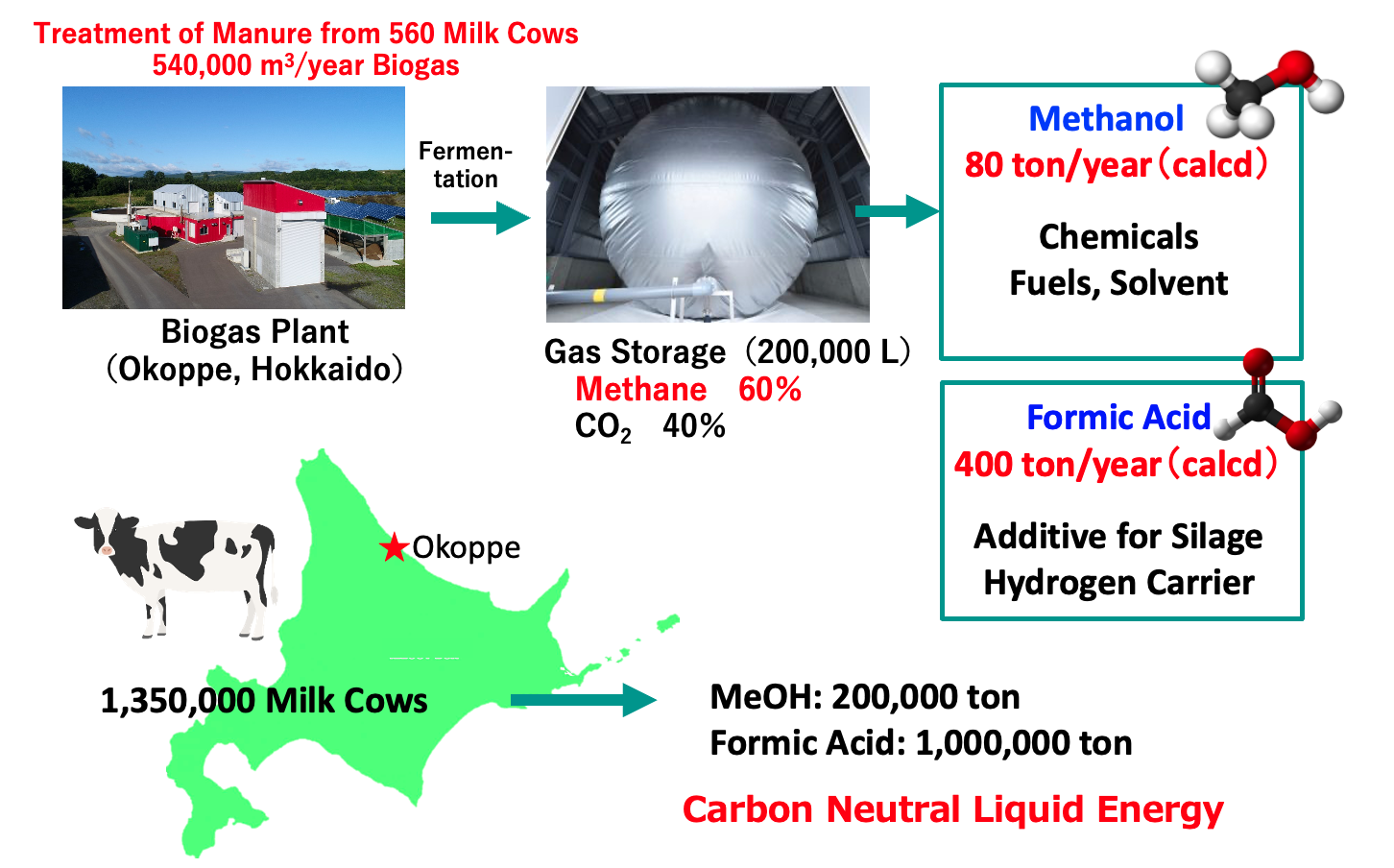
Carbon-neutral circular dairy farming

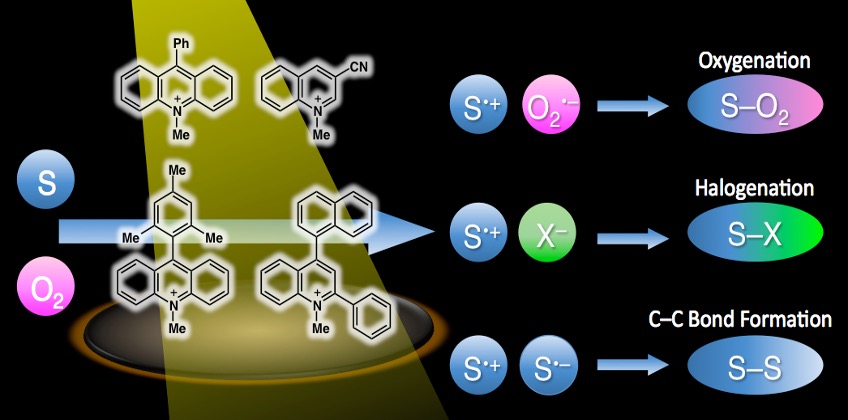
Organic photoredox catalysts and chemical reactions
Lithium-ion-encapsulated fullerene(Li+@C60)
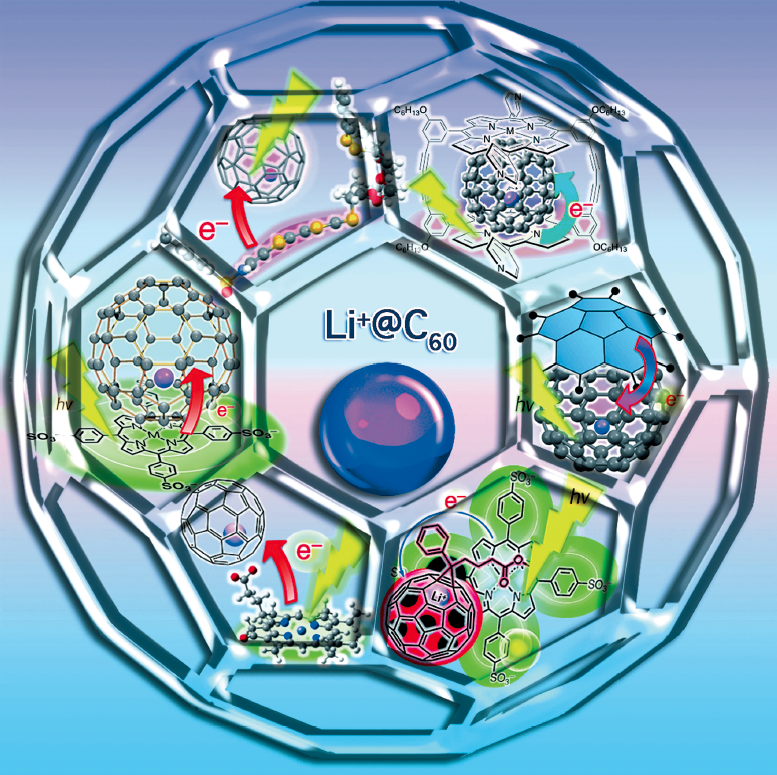
Lithium-ion-encapsulated fullerene (Li+@C60) exhibits greatly enhanced reactivity in photoinduced electrontransfer reduction with electron donors compared with pristine C60. The enhanced reactivity of Li+@C60 results from the more positive one-electron reduction potential of Li+@C60 (+0.14 V versus a standard calomel electrode (SCE)) than that of C60 ( 0.43 V versus SCE), whereas the reorganization energy of electron transfer of Li+@C60 (1.01 eV) becomes larger than that of C60 (0.73 eV) because of the change in electrostatic interactions of encapsulated Li+ upon electron transfer. Li+@C60 can form strong supramolecular complexes with various anionic electron donors through electrostatic interactions. Li+@C60 can also form strong supramolecular p complexes with various electron donors, such as cyclic porphyrin dimers, corannulene, and crown ether fused monopyrrolotetrathiafulvalenes. Photoinduced electron transfer from electron donors to Li+@C60 afforded long-lived chargeseparated states of supramolecular complexes between electron donors and Li+@C60. A photoelectrochemical solar cell composed of supramolecular nanoclusters of Li+@C60 and zinc sulfonated meso-tetraphenylporphyrin exhibits significant enhancement in the photoelectrochemical performance than that of the reference system containing only a single component.
